Medical Device Registration in EAEU
Obtaining an Eurasian Economic Union (EAEU) Registration Certificate
Key Features:
- The EAEU Registration Certificates (RCs) can be extended to the member countries of the EAEU (Russian Federation, Republic of Armenia, Republic of Belarus, Republic of Kazakhstan, Kyrgyz Republic), potentially offering a larger market for manufacturers and distributors of medical devices (MDs). As per the current regulations, the Applicant must select at least one recognition state besides the reference state. A situation where the product registration is valid only in the territory of one EAEU member state (reference state) is possible only if the expert organization of the recognition state does not agree with the expert opinion of the reference state.
- Registration Certificates obtained under EAEU regulations are issued indefinitely.
- EAEU registration imposes stricter requirements on medical devices (MDs) and the registration dossier documents, leading to higher end product quality and better control over the quality management system implementation.
The procedure for MD registration under EAEU rules is regulated by the Decision of the Eurasian Economic Commission Council dated February 12, 2016, No. 46 “On the Rules for Registration and Examination of Safety, Quality, and Efficacy of Medical Devices.”
Medical device registration is carried out in two stages:
Stage 1 – Dossier Preparation:
- Compilation, preparation/adjustment of dossier documents in accordance with the requirements of the Registration Rules and regulatory acts.
At this stage, preliminary consultations with the expert organization on specific registration and examination issues are possible.
Once the list of dossier documents is defined, the conformity of the Medical Device (MD) and its documentation to the general safety and efficiency requirements is confirmed in accordance with the Decision of the Eurasian Economic Commission (EEC) Council No. 27 dated February 12, 2016. For this purpose, the applicable scope of tests for the MD is organized:
-
- Technical testing (in accordance with EEC Council Decision No. 28 dated February 12, 2016).
- Studies to evaluate the biological effect of the MD (in accordance with EEC Council Decision No. 38 dated May 16, 2016).
- Tests for the approval of the type of measuring devices (in accordance with the decisions of the Eurasian Economic Commission Council N 98 dated October 18, 2016, No. 42 dated February 12, 2016).
- Clinical trials (EEC Council Decision No. 29 dated February 12, 2016). The scope, duration, and cost of MD research (testing) are determined by their specifics and depend on their type (implantable or not; active, inactive; sterile, non-sterile; disposable, reusable; in vitro diagnostic devices, etc.).
Our company analyzes, adjusts, and writes documentation for the medical device, determines the required scope of testing, selects the most optimal options for test centers to conduct the tests, and selects the list of required samples and documents for testing. Thus, we manage and accompany the entire testing process: from applying to the test center to receiving the test protocols.
Stage 2 – Registration and Expertise of the MD:
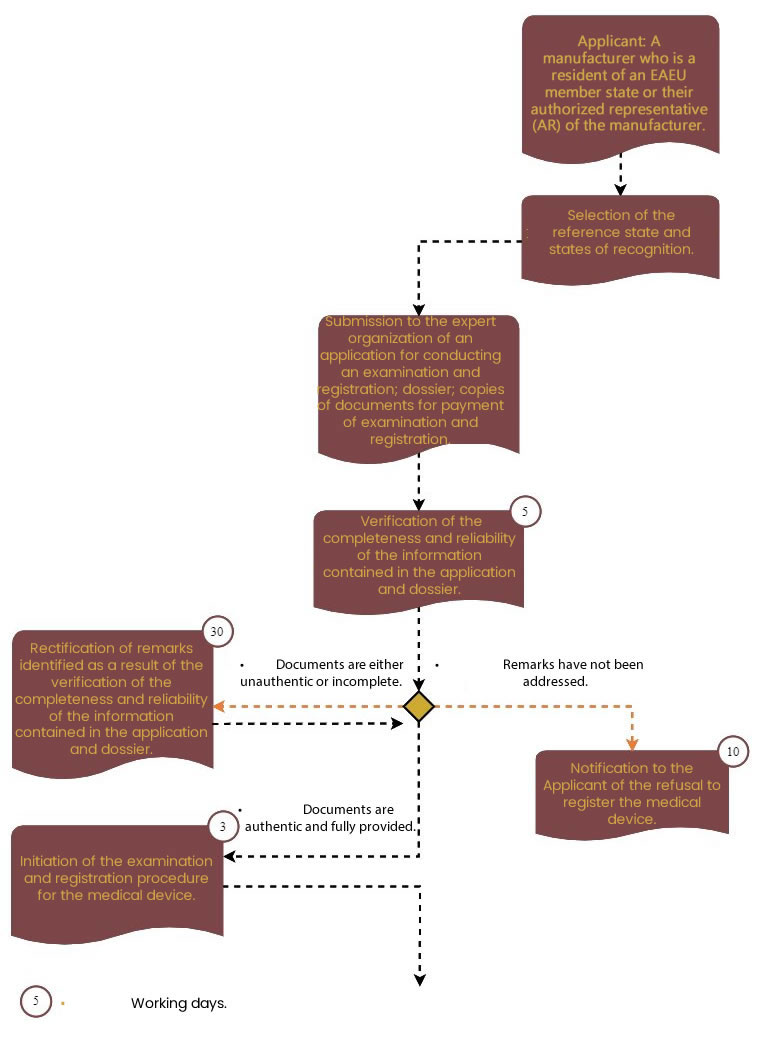
After the document preparation stage, the expertise and registration procedure begins.
The first step involves checking the completeness and accuracy of the documents submitted by the Applicant.
The registration of MDs in the Russian Federation is carried out by the Federal Service for Surveillance in Healthcare (Roszdravnadzor, RZN). In the process of providing the state registration service, RZN sends the dossier for quality, safety, and efficacy expertise to one of the subordinate organizations for expert dossier assessment.
The second stage is carried out in three steps:
Step 1: Submission of documents
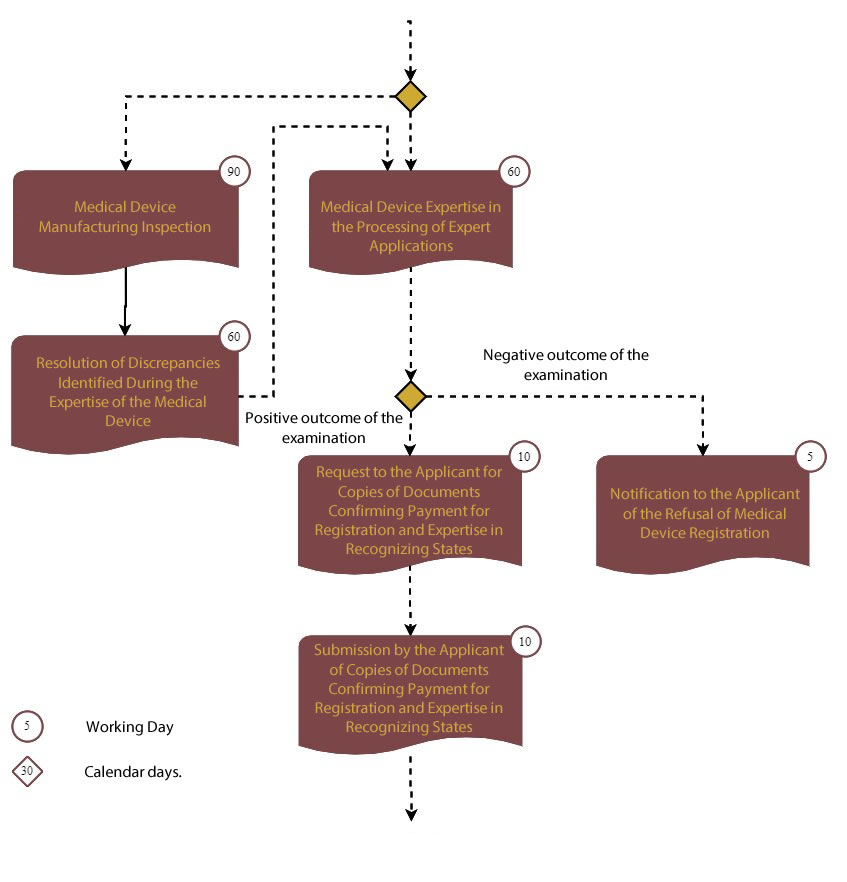
Step 2: Conducting an examination of the medical device
Step 3: Receiving approval of the expert conclusion from the recognition states.
Grounds for Issuance by the Authorized Body (Exceptional Organization) of a Justified Refusal to Register a Medical Device:
a) Non-compliance of the submitted materials and data contained in the registration dossier, quality, efficiency, and safety of the medical device;
b) The risk of applying the device exceeds the potential health benefits in medical practice;
c) failure to rectify identified violations or failure to provide documents upon request.
Upon a positive outcome, the federal service issues the registration certificate and its supplement.
We manage the project at all stages of its consideration, providing professional assistance in dossier preparation, addressing remarks, and preparing reasoned responses to expert services’ inquiries.
Send us a request, and our registration specialists will analyze the data you have, select the optimal registration algorithm, assess the volume of required work for dossier preparation, and send you a commercial offer for the dossier preparation services.
Consultation Request:
To evaluate the cost of registering medical devices, please fill out this brief questionnaire and receive a 10% discount on our services.