Medical Device Registration in Russia
Obtaining a Medical Device Registration Certificate According to Russian Regulations
What is registration and why is it necessary to obtain a registration certificate for medical devices?
In accordance with paragraph 4 of Article 38 of the Federal Law “On the Basics of Health Protection of Citizens in the Russian Federation” dated November 21, 2011, N 323-FZ, the circulation of medical devices registered in the manner established by the Government of the Russian Federation and its authorized federal executive body is permitted in the Russian Federation.
The registration of medical devices (MD) is a state procedure designed to ensure that only high-quality, effective, and safe devices are available on the market. The registration certificate (RC) is a document that confirms the MD’s compliance with established requirements and the fact of their registration in Russia. The RC is a necessary document for the circulation of MDs in the market. In other words, MD registration is a mandatory condition for their import, use, sale, and also production within the territory of the Russian Federation.
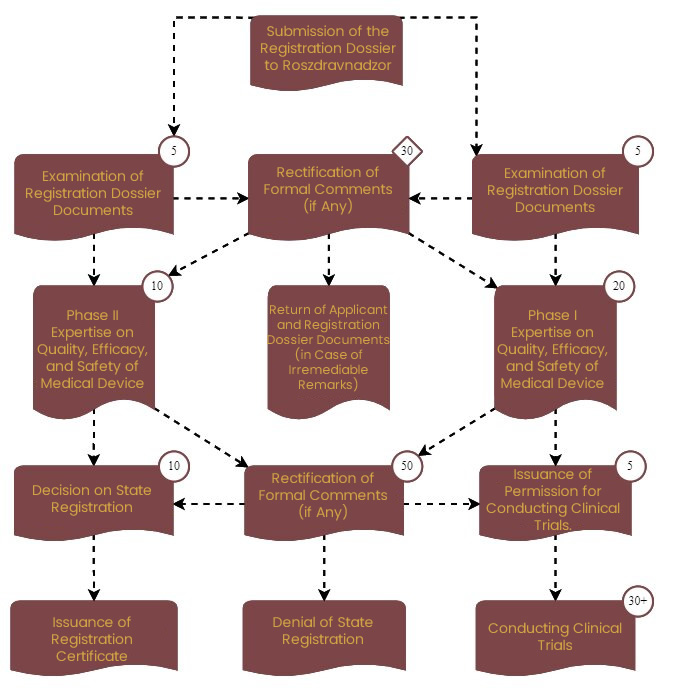
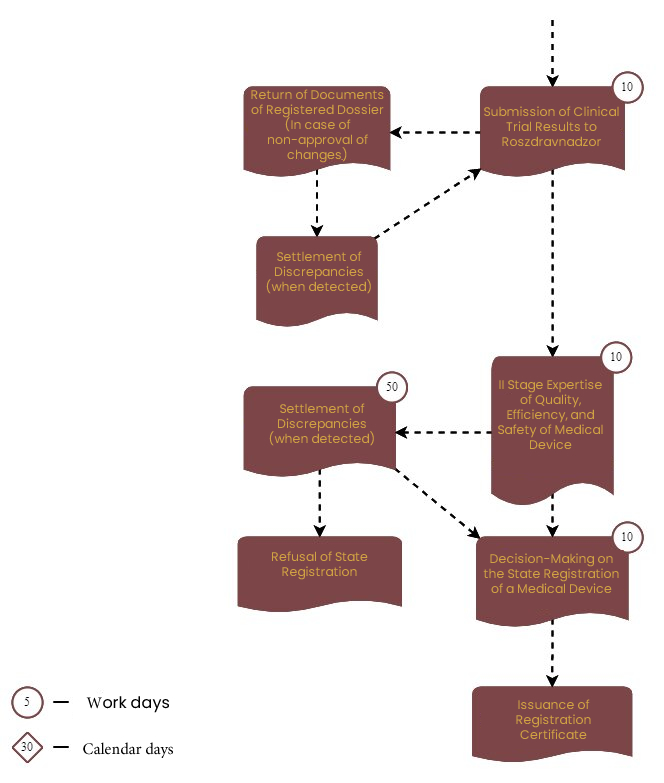
The Federal Service for Surveillance in Healthcare of the Russian Federation (Roszdravnadzor) carries out the registration of MDs in the Russian Federation. During the state registration service process, Roszdravnadzor sends the MD registration dossier for expert assessment of quality, safety, and effectiveness to one of its subordinate organizations.
LLC “SSMK” prepares the registration dossier (RD) in accordance with legislative requirements and registration rules.
Currently, a medical device can also be registered for circulation within the Russian Federation according to:
- The decision of the EEC Council dated February 12, 2016, №46. MDs with an RC obtained according to EAEU rules can circulate in other countries of the Eurasian Economic Union;
- The resolution of the Government of the Russian Federation dated April 3, 2020, №430. This document allows for the registration of devices used in the prevention and fight against COVID-19;
- The resolution of the Government of the Russian Federation dated April 1, 2022, № 552. This regulatory document provides the opportunity to obtain an RC for MDs whose availability to end consumers in the Russian Federation is threatened due to sanctions.
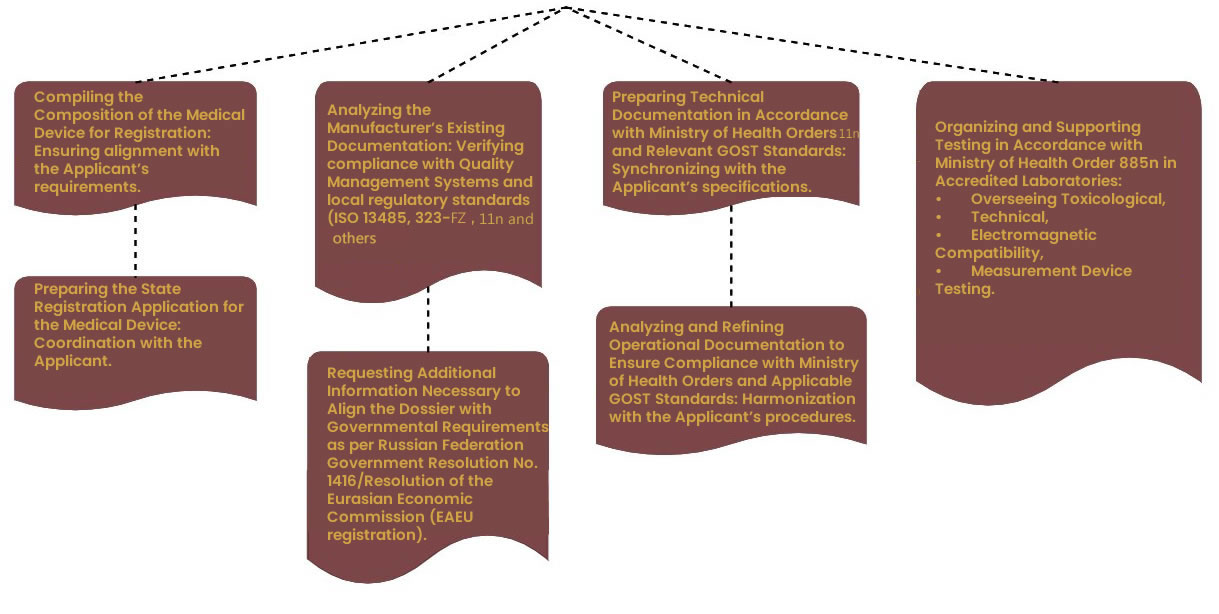
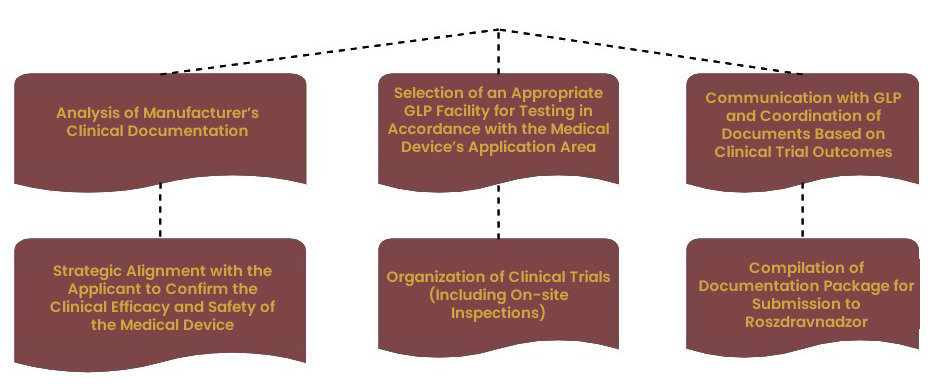
The process of preparing the registration dossier (a set of documents necessary for the registration of a medical device) for a medical device involves comprehensive documentation and adherence to current regulations and standards.
First Stage of Dossier Preparation
Second Stage of Dossier Preparation
The timeframe for obtaining registration certificates for medical devices
The duration for preparing a registration dossier depends on the risk class of the medical device (MD), the chosen registration scheme for the MD, the required volume of testing, and the extent of documentation that needs to be prepared. We register devices within very tight deadlines, as we conduct certain stages of the procedure in parallel where possible, saving your time. The expedited preparation of the registration dossier can take as little as 1.5 months with prompt provision of all required documentation, information, and samples. The average time for preparing the registration dossier is 3-4 months.
The review period for the registration dossier by the ROSZDRAVNADZOR:
Documents for Medical Device Registration
To assemble a registration dossier (a set of documents required for the registration of a medical device), the following documents are needed:
For domestically produced devices:
- Taxpayer Identification Number (INN), Primary State Registration Number (OGRN), and an extract from the Unified State Register of Legal Entities (EGRUL);
- Notarized power of attorney authorizing representation on behalf of the applicant;
- Quality Management System (QMS) compliance certificate, if available;
- Documents proving the existence of a manufacturing facility;
- Technical Specifications (TS), if available (we can develop this document if it’s absent);
- Description of the device to be registered;
- List of devices that need registration;
- Operational documentation, if available (we can develop this document if it’s absent);
- Act of qualification tests, if available;
- Photographs of the device to be registered (we can take these if samples are provided);
- Samples of the medical device (the required quantity of samples is determined based on the types and volume of the required tests).
For foreign-manufactured devices:
- Document registering the manufacturer as a legal entity in the country of manufacture;
- ISO 13485, ISO 9001 certificates, audit report, if available;
- Power of attorney for the manufacturer’s authorized representative in the Russian Federation;
- Documents confirming manufacturing conditions (e.g., business license);
- Technical file, if available (we can develop this document if it’s absent);
- Operational documentation, if available (we can develop this document if it’s absent);
- Photographs of the device to be registered (we can take these if samples are provided);
- Samples of the medical device (the required quantity of samples is determined based on the types and volume of the required tests);
- Reports of conducted tests (test reports);
- Risk management file;
- Reports on medical device validation and verification (reports on packaging stability, software verification, etc. These are requested depending on the requirements for the particular type of MD and are formed during the analysis process);
Depending on the complexity of the medical device, its risk class, and functional characteristics, the list of required documents may change.
Upon a positive outcome, the federal service issues a registration certificate and its annex.
We provide management and professional assistance at all stages of the project, including dossier preparation, addressing remarks, and preparing substantiated responses to expert services inquiries.
Please send us your inquiry. Our registration specialists will analyze your data, select an optimal registration algorithm, evaluate the volume of work required for dossier preparation, and send you a commercial offer for these services.
Consultation Request:
To evaluate the cost of registering medical devices, please fill out this brief questionnaire and receive a 10% discount on our services.